MENTHOL -- MOLECULES OF TASTE
|
| Menthol | |
|---|---|
 |
|
| General | |
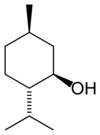
| Systematic name | 2-(2-Propyl)-5-methyl- 1-cyclohexanol |
| for racemic (−)-isomer | (2R)-(2-Propyl)-(5S)-methyl- (1R)-cyclohexanol |
| Other names | 3-p-Menthanol, Hexahydrothymol, Menthomenthol, peppermint camphor |
| Molecular formula | C10H20O |
| SMILES | CC1CCC(C(C1)O)C(C)C |
| Molar mass | 156.27 g/mol |
| Appearance | White or colorless crystalline solid |
| CAS number | [89-78-1], racemic [2216-51-5], (−)-isomer |
| Properties | |
| Density and phase | 0.890 g/cm3, solid (racemic or (−)-isomer) |
| Solubility in water | Slightly soluble, (−)-isomer |
| In ethanol, diethyl ether, acetone, chloroform acetic acid, hexane |
Soluble |
| Melting point | 36-38 °C (311 K), racemic 42-45 °C (318 K), (−)-form (α) 35-33-31 °C, (−)-isomer |
| Boiling point | 212 °C (485 K) |
| Chiral rotation | -50° at 18 °C, 10% EtOH soln. |
| Hazards | |
| Main hazards | Irritant, flammable |
| Flash point | 93 °C |
| RTECS number | OT0350000, racemic OT0700000, (−)-enantiomer |
| Related compounds | |
| Related alcohols | Cyclohexanol, Pulegol, Dihydrocarveol, Piperitol |
| Related compounds | Menthone, Menthene, Thymol, p-Cymene, Citronellal |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
Menthol is a covalent organic compound made synthetically or obtained from peppermint or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (-)-menthol, which is assigned the (1R,2S,5R) configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation.
History and occurrence
There is evidence[1] that menthol has been known in Japan for more than 2000 years, but in the west it was not isolated until 1771, by Gambius.[2] (-)-Menthol (also called l-menthol or (1R,2S,5R)-menthol) occurs naturally in peppermint oil (along with a little menthone, the ester menthyl acetate and other compounds), obtained from mentha x piperita. Japanese menthol also contains a small percentage of the 1-epimer, (+)-neomenthol.
Applications
Menthol is included in many products for a variety of reasons. These include:
- In non-prescription products for short-term relief of minor sore throat and minor mouth or throat irritation
- Examples: lip balms and cough medicines
- As an antipruritic to reduce itching
- As a topical analgesic to relieve minor aches and pains such as muscle cramps, sprains, headaches and similar conditions, alone or combined with products like Camphor or Capsicum. In Europe it tends to appear as a gel or a cream, while in the US patches and body sleeves are very frequently used
- Examples: Tiger Balm IcyHot patches or knee/elbow sleeves
- In decongestants for chest and sinuses (cream, patch or nose inhaler)
- Examples: Vicks Vaporub
- In certain medications used to treat sunburns, as it provides a cooling sensation (then often associated with Aloe)
- As an additive in certain cigarette brands, for flavor, to reduce the throat and sinus irritation caused by smoking and arguably to reduce the bad-breath smokers experience and possibly improve the smell of second-hand smoke.
- Commonly used in oral hygiene products and bad-breath remedies like mouthwash, toothpaste, mouth and tongue-spray, and more generally as a food flavor agent e.g. in chewing-gum, candy
- In a soda as well as in a syrup to be mixed with water to obtain a very low alcohol drink or (brand Rickles in France). The syrup is/was also used to alleviate nausea, in particular motion sickness, by pouring a few drops on a lump of sugar.
- As a pesticide against tracheal mites of honeybees
- In perfumery, menthol is used to prepare menthyl esters to emphasise floral notes (especially rose)
- In first aid products such as "mineral ice" to produce a cooling effect as a substitute for real ice in the absence of water or electricity (Pouch, Body patch/sleeve or cream)
- In various patches ranging from fever-reducing patches applied to children's foreheads to "foot patches" to relieve numerous ailments (the latter being much more frequent and elaborate in Asia, esp. Japan: some variety use `functional protrusion' i.e. small bumps to massage ones feet as well as soothing them and cooling them down)
- In some beauty products such as hair-conditioners, based on natural ingredients (ex: St Ives)
Some supporters of the homeopathic theory of pharmacology believe that menthol interferes with the effects of homeopathic remedies. Its use is strongly discouraged for those seeking homeopathic cures, to the point of prohibiting the use of mint-flavored toothpaste. Currently no other reported nutrient or herb interactions involve menthol. Menthol is available as a dietary supplement or natural medicine in the form of peppermint oil. It is used in Eastern medicine to treat indigestion, nausea, sore throat, diarrhoea, colds, and headaches. (-)-Menthol has low toxicity: Oral (rat) LD50: 3300 mg/kg; Skin (rabbit) LD50: 15800 mg/kg).
Chemical properties
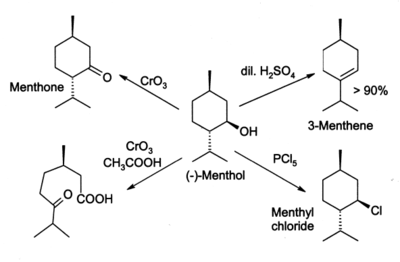
Menthol reacts in many ways like a normal secondary alcohol. It is oxidised to menthone by oxidising agents such as chromic acid, though under some conditions the oxidation can go further and break open the ring. Menthol is easily dehydrated to give mainly 3-menthene, by the action of 2% sulfuric acid. PCl5 gives menthyl chloride.
Biological properties
Menthol's ability to chemically trigger cold-sensitive receptors in the skin is responsible for the well known cooling sensation that it provokes when inhalated, eaten, or applied to the skin. Menthol does not cause an actual drop in temperature. [3]. In this sense it is similar to capsaicin, the chemical responsible for the spiciness of hot peppers (which stimulates heat sensors, also without causing actual temperature rise).
Notes
- J. L. Simonsen, The Terpenes Volume I (2nd edition), Cambridge University Press, 1947, p230-249.
- Adversoriorum varii argumentii, Liber unus, Leiden, 1771, p99.
- R. Eccles, Menthol and Related Cooling Compounds, Journal of Pharm. Pharmacol. 1994 46: 618-630
References
- E. E. Turner, M. M. Harris, Organic Chemistry, Longmans, Green & Co., London, 1952.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
Tastant Molecules
Sugars
Artificial Sweeteners
Bitter Tastants
Umami
Acids
Thermal