MOLECULES OF TASTE -- GLUCOSE
 |
Glucose (Glc), a monosaccharide (or simple sugar), is the most important carbohydrate in biology. The cell uses it as a source of energy and metabolic intermediate. Glucose is one of the main products of photosynthesis and starts cellular respiration in both prokaryotes and eukaryotes.
Two isomers of the aldohexose sugars are known as glucose, only one of which (D-glucose) is biologically active. This form (D-glucose) is often referred to as dextrose (dextrose monohydrate), especially in the food industry. This article deals with the D-form of glucose. The mirror-image of the molecule, L-glucose, cannot be used by cells.
| Glucose | |
|---|---|
| Chemical name | 6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| Synonym for D-glucose | dextrose |
| Varieties of D-glucose | α-D-glucose; β-D-glucose |
| Abbreviations | Glc |
| Chemical formula | C6H12O6 |
| Molecular mass | 180.16 g mol−1 |
| Melting point | α-D-glucose: 146°C β-D-glucose: 150°C |
| Density | 1.54 g cm−3 |
| CAS number | 50-99-7 (D-glucose) |
| CAS number | 921-60-8 (L-glucose) |
| SMILES | C(C1C(C(C(C(O1)O)O)O)O)O |
Structure
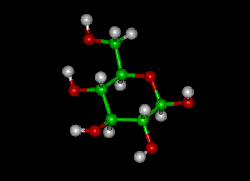
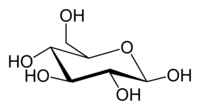
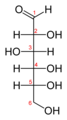
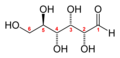
Glucose (C6H12O6) contains six carbon atoms and an aldehyde group and is therefore referred to as an aldohexose. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form(in equilibrium), the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In water solution both forms are in equilibrium, and at pH 7 the cyclic one is the predominant. As the ring contains five carbon atoms and one oxygen atom, which resembles the structure of pyran, the cyclic form of glucose is also referred to as glucopyranose. In this ring, each carbon is linked to an hydroxyl side group with the exception of the fifth atom, which links to a sixth carbon atom outside the ring, forming a CH2OH group.
Isomers
Aldohexose sugars have 4 chiral centers giving 24 = 16 optical stereoisomers. These are split into two groups, L and D, with 8 sugars in each. Glucose is one of these sugars, and L and D-glucose are two of the stereoisomers. Only 7 of these are found in living organisms, of which D-glucose (Glu), D-galactose (Gal) and D-mannose (Man) are the most important. These eight isomers (including glucose itself) are all diastereoisomers in relation to each other and all belong to the D-series.
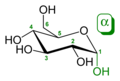
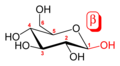
An additional asymmetric center at C-1 (called the anomeric carbon atom) is created when glucose cyclizes and two ring structures, called anomers, can be formed — α-glucose and β-glucose. They differ structurally in the orientation of the hydroxyl group linked to C-1 in the ring. When D-glucose is drawn as a Haworth projection, the designation αmeans that the hydroxyl group attached to C-1 is below the plane of the ring, β means it is above. The α and β forms interconvert over a timescale of hours in aqueous solution, to a final stable ratio of α:β 36:64, in a process called mutarotation.
 |
 |
 |
 |
Production
Natural
- Glucose is one of the products of photosynthesis in plants and some prokaryotes.
- In animals and fungi, glucose is the result of the breakdown of glycogen, a process known as glycogenolysis. In plants - the breakdown substrate is starch.
- In animals, glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate and glycerol, by a process known as gluconeogenesis.
Commercial
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat, potato, cassava, arrowroot, and sago are all used in various parts of the world. In the United States, cornstarch (from maize) is used almost exclusively.
This enzymatic process has two stages. Over the course of 1-2 hours near 100 °C, these enzymes hydrolyze starch into smaller carbohydrates containing on average 5-10 glucose units each. Some variations on this process briefly heat the starch mixture to 130 C or hotter one or more times. This heat treatment improves the solubility of starch in water, but deactivates the enzyme, and fresh enzyme must be added to the mixture after each heating.
In the second step, known as "saccharification", the partially hydrolyzed starch is completely hydrolyzed to glucose using the glucoamylase enzyme from the fungus Aspergillus niger. Typical reaction conditions are pH 4.0-4.5, 60 °C, and a carbohydrate concentration of 30-35% by weight. Under these conditions, starch can be converted to glucose at 96% yield after 14 days. Still higher yields can be obtained using more dilute solutions, but this approach requires larger reactors and processing a greater volume of water, and is not generally economical. The resulting glucose solution is then purified by filtration and concentrated in a multiple-effect evaporator. Solid D-glucose is then produced by repeated crystallizations.
Function
We can speculate on the reasons why glucose, and not another monosaccharide such as fructose (Fru) , is so widely used in evolution/the ecosystem/metabolism. Glucose can form from formaldehyde under abiotic conditions, so it may well have been available to primitive biochemical systems. Probably more important to advanced life is the low tendency of glucose, by comparison to other hexose sugars, to non-specifically react with the amino groups of proteins. This reaction (glycation) reduces or destroys the function of many enzymes. The low rate of glycation is due to glucose's preference for the less reactive cyclic isomer. Nevertheless, many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. In contrast, enzyme-regulated addition of glucose to proteins by glycosylation is often essential to their function.
As an energy source
Glucose is a ubiquitous fuel in biology. It is used as an energy source in most organisms, from bacteria to humans. Use of glucose may be by either aerobic or anaerobic respiration (fermentation). Carbohydrates are the human body's key source of energy, through aerobic respiration, providing approximately 4 kilocalories (17 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g. starch) yields mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the Citric acid cycle (TCAC), glucose is oxidized to eventually form CO2and water, yielding energy, mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. A high fasting blood sugar level is an indication of prediabetic and diabetic conditions.
Glucose in glycolysis
Use of glucose as an energy source in cells is via aerobic or anaerobic respiration. Both of these start with the early steps of the glycolysis metabolic pathway. The first step of this is the phosphorylation of glucose by hexokinase to prepare it for later breakdown to provide energy.
The major reason for the immediate phosphorylation of glucose by a hexokinase is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. Irreversible first steps of a metabolic pathway are common for regulatory purposes.
As a precursor
Glucose is critical in the production of proteins and in lipid metabolism. Also, in plants and most animals, it is a precursor for vitamin C (ascorbic acid) production. It is modified for use in these processes by the glycolysis pathway. Glucose is used as a precursor for the synthesis of several important substances. starch soulution Starch, cellulose, and glycogen ("animal starch") are common glucose polymers (polysaccharides). Lactose, the predominant sugar in milk, is a glucose-galactose disaccharide. In sucrose, another important disaccharide, glucose is joined to fructose. These synthesis processes also rely on the phosphorylation of glucose by the first step of glycolysis.
Sources and absorption
All major dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or together with another monosaccharide, as in sucrose and lactose. In the lumen of the duodenum and small intestine the oligo- and polysaccharides are broken down to monosaccharides by the pancreatic and intestinal glycosidases. Glucose is then transported across the apical membrane of the enterocytes by SLC5A1 and later across their basal membrane by SLC2A2 (ref). Some of glucose goes directly to fuel brain cells and erythrocytes, while the rest makes its way to the liver and muscles, where it is stored as glycogen, and to fat cells, where it is stored as fat. Glycogen is the body's auxiliary energy source, tapped and converted back into glucose when there is need for energy.
Tastant Molecules
Sugars
Artificial Sweeteners
Bitter Tastants
Umami
Acids
Thermal
