CHEMICAL AND PHYSICAL PROPERTIES OF ASPARTAME
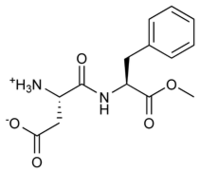
Aspartame is the name for an artificial, non-carbohydrate sweetener, aspartyl-phenylalanine-1-methyl ester; i.e., the methyl ester of the dipeptide of the amino acids aspartic acid and the essential amino acid phenylalanine.
| Aspartame | |
|---|---|
 |
|
| Chemical name | N-(L-α-Aspartyl)-L-phenylalanine, 1-methyl ester |
| Other names | Aspartame NutraSweet Canderel Equal |
| Chemical formula | C14H18N2O5 |
| Molecular mass | 294.301 g/mol |
| CAS number | [22839-47-0] |
| Melting point | 246-247 °C |
| Boiling point | decomposes |
| SMILES | [NH3+] [C@@H](CC([O-])=O)C(N[C@@H] (CC1=CC=CC=C1)C(OC)=O)=O |
| NFPA 704 | |
| Disclaimer and references | |
This sweetener is marketed under a number of trademark names, such as Equal, NutraSweet, and Canderel, and is an ingredient of approximately 6,000 consumer foods and beverages sold worldwide. It is commonly used in diet soft drinks, and is often provided as a table condiment. It is also used in some brands of chewable vitamin supplements and common in many sugar-free chewing gums. However, aspartame is not always suitable for baking, because it often breaks down when heated and loses much of its sweetness. In the European Union, it is also known under the E number (additive code) E951. Aspartame is also one of the sugar substitutes used by diabetics. Nonetheless, aspartame has been a subject of a vigorous public controversy. (See: Aspartame Controversy.)
Chemistry
Aspartame is the methyl ester of the dipeptide of the natural amino acids L-aspartic acid and L-phenylalanine. Under strongly acidic or alkaline conditions, aspartame first generates methanol by hydrolysis. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids.
Discovery and approval
Aspartame was discovered in 1965 by James M. Schlatter, a chemist working for G.D. Searle & Company. Schlatter had synthesized aspartame in the course of producing an anti-ulcer drug candidate. He discovered its sweet taste serendipitously when he licked his finger, which had accidentally become contaminated with aspartame.
Following initial safety testing, there was debate as to whether these tests had indicated that aspartame may cause cancer in rats; as a result, the U.S. Food and Drug Administration (FDA) did not approve its use as a food additive in the United States for many years. In 1980, the FDA convened a Public Board of Inquiry (PBOI) consisting of independent advisors charged with examining the purported relationship between aspartame and brain cancer. The PBOI concluded that aspartame does not cause brain damage, but it recommended against approving aspartame at that time, citing unanswered questions about cancer in laboratory rats. In 1981, U.S. President Ronald Reagan appointed Arthur Hull Hayes as FDA commissioner. Citing data from a Japanese study that had not been available to the members of the PBOI, Hayes approved aspartame for use in dry goods.[2]In 1983 FDA further approved aspartame for use in carbonated beverages, and for use in other beverages, baked goods, and confections in 1993. In 1996, the FDA removed all restrictions from aspartame allowing it to be used in all foods.
In 1985, G.D. Searle was purchased by Monsanto. In this acquisition, Searle’s aspartame business became a separate Monsanto subsidiary, the NutraSweet Company. Monsanto subsequently sold the NutraSweet company to J.W. Childs Equity Partners II L.P. on May 25, 2000.[3] The U.S. patent on aspartame expired in 1992, and the aspartame market is now hotly contested between the NutraSweet Company and other manufacturers such as Ajinomoto, Merisant and the Holland Sweetener Company — the latter of which is exiting the business in the fourth quarter of 2006 due to a "persistently unprofitable business position" because "global aspartame markets are facing structural oversupply, which has caused worldwide strong price erosion over the last 5 years."[4]
Properties and use
Aspartame attractiveness as a sweetener comes from the fact that it is approximately 180 times sweeter than sugar in typical concentrations without the high energy value of sugar. While aspartame, like other peptides, has a caloric value of 4 kilocalories (17 kilojoules) per gram, the quantity of aspartame needed to produce a sweet taste is so small that its caloric contribution is negligible, which makes it a popular sweetener for those trying to avoid calories from sugar. The taste of aspartame is not identical to that of sugar: aspartame’s sweetness has a slower onset and longer duration than sugar™, and some consumers find it unappealing. Blends of aspartame with acesulfame potassium are purported to have a more sugar-like taste, and to be more potent than either sweetener used alone.
Like many other peptides, aspartame may hydrolyze (break down) into its constituent amino acids under conditions of elevated temperature or high pH. This makes aspartame undesirable as a baking sweetener, and prone to degradation in products hosting a high-pH, as required for a long shelf life. Aspartame’s stability under heating can be improved to some extent by encasing it in fats or in maltodextrin. Aspartame’s stability when dissolved in water depends markedly on pH. At room temperature, it is most stable at pH 4.3, where its half-life is nearly 300 days. At pH 7, however, its half-life is only a few days. Most soft-drinks have a pH between 3 and 5, where aspartame is reasonably stable. In products that may require a longer shelf life, such as syrups for fountain beverages, aspartame is sometimes blended with a more stable sweetener, such as saccharin.
In products such as powdered beverages, aspartame’s amino group can undergo a Maillard reaction with the aldehyde groups present in certain aroma compounds. The ensuing loss of both flavor and sweetness can be prevented by protecting the aldehyde as an acetal.
Metabolism
Upon ingestion, aspartame breaks down into several constituent chemicals, including aspartic acid, phenylalanine, methanol, and further breakdown products including formaldeyhyde[5] and formic acid. There is some controversy surrounding the rate of breakdown into these various products and the effects that they have on those that consume aspartame-sweetened foods (see Aspartame Controversy, below).
The naturally occurring essential amino acid phenylalanine is a health hazard to those born with phenylketonuria (PKU), a rare inherited disease that prevents the essential amino acid phenylalanine from being properly metabolized. Because of this, phenylalanine can accumulate in the body and cause health problems including mental retardation. People with PKU are placed on a special diet with a severe restriction of phenylalanine from birth to adolescence or after. Women with PKU must remain on the diet throughout pregnancy. Since individuals with PKU must consider aspartame as an additional source of phenylalanine, aspartame-containing foods sold in the United States must state "Phenylketonurics: Contains Phenylalanine" on their product labels.
Aspartame controversy
-
Main article: Aspartame controversy
Aspartame has been the subject of a vigorous public controversy regarding its safety and the circumstances around its approval. Some studies have also recommended further investigation into connections between aspartame and diseases such as brain tumors, brain lesions, and lymphoma. [6] [7] [8] These findings, combined with notable conflicts of interest in the approval process, have engendered vocal activism regarding the possible risks of aspartame.[9] [10]
References
- Merck Index, 11th Edition, 861.
- http://archive.gao.gov/d28t5/133460.pdf
- http://www.findarticles.com/p/articles/mi_m0EUY/is_22_6/ai_6292082 http://www.marketwire.com/mw/release_html_b1?release_id=115447
- Trocho, C.; Pardo, R.; Rafecas, I.; Virgili, J.; Remesar, X.; Fernandez-Lopez, J.A.; Alemany, M. Formaldehyde derived from dietary aspartame binds to tissue components in vivo., Life Sci., 1998, 63(5), 337-349; Abstract
- Olney, J.W., N.B. Farber, E. Spitznagel, L.N. Robins, 1996. "Increasing Brain Tumor Rates: Is There a Link to Aspartame?" Journal of Neuropathology and Experimental Neurology, Volume 55, pages 1115-1123.
- Soffritti, Morando, et al., "First Experimental Demonstration of the Multipotential Carcinogenic Effects of Aspartame Administered in the Feed to Sprague-Dawley Rats," Environmental Health Perspectives, Volume 114(3):379-385, 2006. http://www.ehponline.org/members/2005/8711/8711.pdf. Roberts, H.J., "Does Aspartame Cause Human Brain Cancer," Journal of Advancement in Medicine, Volume 4(4):231-241, 1991.
- GAO 1986. "Six Former HHS Employees' Involvement in Aspartame's Approval," United States General Accounting Office, GAO/HRD-86-109BR, July 1986. http://archive.gao.gov/d4t4/130780.pdf
- Gordon, Gregory, United Press International Investigation, "NutraSweet: Questions Swirl," 1987. http://www.dorway.com/upipaper.txt
Tastant Molecules
Sugars
Artificial Sweeteners
Bitter Tastants
Umami
Acids
Thermal
