MOLECULES OF TASTE --PIPERINE
Piperine Molecule in Black Pepper
Piperine s the alkaloid responsible for the pungency of black pepper and long pepper, along with chavicine (an isomer of piperine). It has also been used in some forms of traditional medicine and as an insecticide. Piperine forms monoclinic needles, is slightly soluble in water (40 mg/L, or 1g/25L (18°C)) and more so in alcohol (1g/15mL), ether (1g/36mL) or chloroform (1g/1.7mL): the solution in alcohol has a pepper-like taste. It yields salts only with strong acids. The platinichloride B4•H2PtCl6 forms orange-red needles. ("B" denotes one mole of the alkaloid base in this and the following formulae.) Iodine in potassium iodide added to an alcoholic solution of the base in presence of a little hydrochloric acid gives a characteristic periodide, B2•HI•I2, crystallising in steel-blue needles, mp. 145°C. Anderson[2] first hydrolysed piperine by alkalis into a base and an acid, which were later named[3] piperidine and piperic acid respectively. The alkaloid was synthesised[4] by the action of piperoyl chloride on piperidine.
 |
 |
|
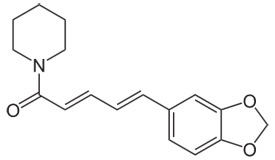
Piperine Chemical Structure |
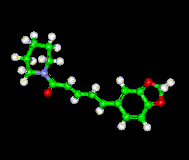
Ball and Stick Model of Piperine |
| Piperine | |
|---|---|
IUPAC name
1-[5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine |
|
Other names
5-(3,4-Methylenedioxyphenyl)-2,4-pentadienoyl-2-piperidine Piperoylpiperidine
|
|
| Identifiers | |
| CAS number | 94-62-2 |
| PubChem | 638024 |
| ChemSpider | 553590 |
| UNII | U71XL721QK |
| ChEMBL | CHEMBL43185 |
| Jmol-3D images | |
| Properties | |
| Molecular formula | C17H19NO3 |
| Molar mass | 285.34 g mol-1 |
| Density | 1.193 g/cm3 |
| Melting point | 130 °C; 266 °F; 403 K |
| Boiling point | decomposes |
| Hazards | |
| MSDS | MSDS for piperine |
Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) |
|
Heat |
(SCOVALE SCALE: 100,000) |
|---|---|
Preparation
Piperine is commercially available. If desired, it may be extracted from black pepper using dichloromethane.[5] Aqueous hydrotopes can also be used in the extraction to result in high yield and selectivity.[6] The amount of piperine varies from 1-2% in long pepper, to 5-10% in commercial white and black peppers.[7] Further, it may be prepared by treating the solvent-free residue from an alcoholic extract of black pepper, with a solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine) and solution of the washed, insoluble residue in warm alcohol, from which the alkaloid crystallises on cooling.[8]
Biological activity
Piperine was discovered in 1819 by Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum, the source plant of both the black and white pepper grains.[9] Piper longum and Piper officinarum (Miq.) C. DC. (=Piper retrofractum Vahl),[vague] two species called "long pepper" also were found to contain it by Flückiger and Hanbury.[10] West African pepper also contains it.[11]
The pungency of capsaicin and piperine is caused by activation of the heat and acidity sensing TRPV ion channel TRPV1 on nociceptors (pain sensing nerve cells).[12]
Piperine has been found to inhibit human CYP3A4 and P-glycoprotein, enzymes important for the metabolism and transport of xenobiotics and metabolites.[13] In animal studies, piperine also inhibited other enzymes important in drug metabolism.[14][15] By inhibiting drug metabolism, piperine may increase the bioavailability of various compounds and alter the effectiveness of some medications.[14] Notably, piperine may enhance bioavailability of curcumin by 2000% in humans.[16] The exact mechanism of piperine's bioavailability enhancing abilities is unknown.[17] Chemopreventive efficacy of curcumin and piperine has been shown during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis.[18]
Recently the journal Molecular Nutrition Food Research published evidence that piperine can enhance the pharmacokinetic parameters of resveratrol via inhibiting its glucuronidation, thereby slowing its elimination. In February 2008, researchers discovered that piperine can stimulate pigmentation in the skin, together with the exposure to UVB light.[19][20] Piperine has shown 'anti-depression like activity', and cognitive enhancing effects in rats.[21 Piperine has shown anti-inflammatory and anti-arthritic effects in human interleukin-1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models.[22]
References
- Merck Index, 11th Edition, 7442
- Annalen, 1850, 75, 82; 84, 345, cf. Wertheim and Rochleder, ibid., 1845, 54, 255.
- Babo & Keller, Journ. pr. chem., 1857, 72, 53.
- Rugheimer, Ber., 1882, 15, 1390.
- Epstein, William W.; Netz, David F.; Seidel, Jimmy L. (1993). "Isolation of piperine from black pepper". J. Chem. Ed. 70 (7): 598. doi:10.1021/ed070p598.
- Gaikar. Process for extraction of piperine from piper species. US 6365601, April 2, 2002.
- http://www.tis-gdv.de/tis_e/ware/gewuerze/pfeffer/pfeffer.htm#selbsterhitzung
- Ikan, Raphael (1991). Natural Products: A Laboratory Guide 2nd Ed.. San Diego: Academic Press, Inc. p. 223-224.
- Oersted, "Über das Piperin, ein neues Pflanzenalkaloid" [On piperine, a new plant alkaloid], (Schweigger's) Journal für Chemie und Physik, vol. 29, no. 1, pages 80-82 (1820).
- Pharmacographia (London: Macmillan & Co., 1879), p. 584.
- Stenhouse in Pharm. J., 1855, 14, 363.
- J. Pharmacol. 144 (6): 781–90. doi:10.1038/sj.bjp.0706040. PMC 1576058. PMID 15685214.
- Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF (August 2002). "Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4". J. Pharmacol. Exp. Ther. 302 (2): 645–50. doi:10.1124/jpet.102.034728. PMID 12130727.
- a,b -- Atal CK, Dubey RK, Singh J (January 1985). "Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism". J. Pharmacol. Exp. Ther. 232 (1): 258–62. PMID 3917507.
- Reen RK, Jamwal DS, Taneja SC, et al. (July 1993). "Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine". Biochem. Pharmacol. 46 (2): 229–38. doi:10.1016/0006-2952(93)90408-O. PMID 8347144.
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (May 1998). "Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers". Planta Med. 64 (4): 353–6. doi:10.1055/s-2006-957450. PMID 9619120.
- Majeed, M. Use of piperine as a bioavailability enhancer. US Patent 5744161, October 26, 1999.
- Manoharan S, Balakrishnan S, Menon V, et al. Singapore Med J. 2009 Feb;50(2):139-46.
- Faas, L.; Venkatasamy, R.; Hider, R. C.; Young, A. R.; Soumyanath, A. (2008). "In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model". British Journal of Dermatology 158 (5): 941–50. doi:10.1111/j.1365-2133.2008.08464.x. PMID 18284389.
- "Pepper 'to treat pigment disease'". BBC News. 2008-02-14.
- Wattanathorna, Jintanaporn; Pennapa Chonpathompikunlertb, Supaporn Muchimapuraa, Aroonsri Pripremc, Orathai Tankamnerdthai (September 2008). "Piperine, the potential functional food for mood and cognitive disorders". Food and Chemical Toxicology 46 (9): 3106–3110. doi:10.1016/j.fct.2008.06.014. PMID 18639606.
- Bang JS, Oh DH, Choi HM, et al. Arthritis Res Ther. 2009 Mar 30;11(2):R49.
Tastant Molecules
Sugars
Artificial Sweeteners
Bitter Tastants
Umami
Acids
Thermal
